Rare Pulmonology News
Advertisement
Spotlight On
Activated PI3K Delta Syndrome (APDS)
Activated PI3K Delta Syndrome (APDS) is a rare, genetic primary immunodeficiency
Rare View
Activated PI3K delta syndrome, known as APDS (previously known as PASLI* Disease) is a rare primary immunodeficiency, first discovered in 2013. APDS is caused by genetic variants in either one of two genes known as PIK3CD or PIK3R1, which encode proteins that are vital to the normal development and function of immune cells. Signs and symptoms of APDS start in childhood, and patients are vulnerable to repeat infections and immune dysregulation such as lymphadenopathy, splenomegaly, autoimmune cytopenias, and even lymphoma.†
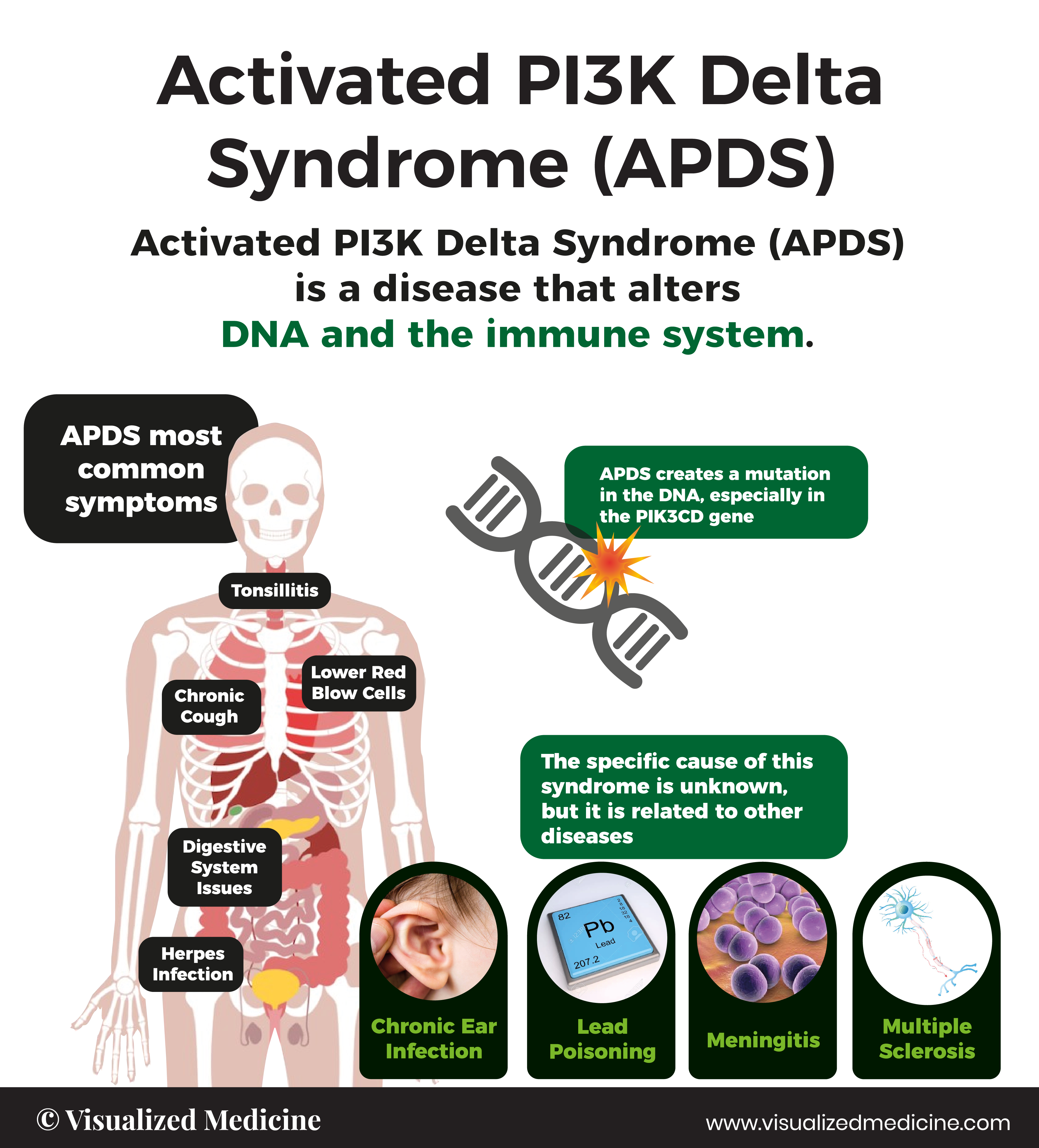
5 Facts you should know
FACT
Since APDS was only recently fully characterized and shares many features of other immune disorders, patients with APDS may have been previously misdiagnosed with other conditions.
FACT
Signs and symptoms of APDS start in childhood, and patients are vulnerable to repeat infections and immune dysregulation such as severe respiratory tract infections, sinusitis, swollen lymph nodes, like tonsilitis and nodules in the air ways.
FACT
Recurrent respiratory infections affect virtually all APDS patients.
FACT
Bronchiectasis is a severe and common complication - lung infections affect up to 60% of APDS1 patients.
FACT
Genetic testing is the only way to definitively diagnose APDS and other primary immunodeficiencies.
Interest Over Time
Google searches
Common Signs & Symptoms
Bronchiectasis
Permanent enlargement of the airways of the lungs
Decreased circulating IgG2 level
Decreased proportion of class-switched memory B cells
Decreased specific pneumococcal antibody level
Increased circulating IgM level
Increased proportion of transitional B cells
Lymphadenopathy
Recurrent ear infections
† All About APDS https://allaboutapds.com
Caregiver Corner
Tools and resources for your patients living with LGS

Comprehensive Care Centers
If you or someone you care for suspects they may have APDS, or you’ve been given an APDS diagnosis, it’s important to find a physician who is familiar with diagnosing and managing patients with APDS. This tool features experts who can support patients through diagnosis, disease management and genetic testing. Click below to find a physician in your area.
An overview of Activated PI3K Delta Syndrome (APDS) and primary immunodeficiencies

Help your patients learn about APDS and primary immunodeficiencies. Get years of knowledge and experience condensed into a simple report.
Other resources
Click the drop-down to see info on Patient Advocacy Groups and a Glossary of common terms
- Immune Deficiency Foundation (IDF) IDF is a national nonprofit organization dedicated to improving the diagnosis, treatment, and quality of life of individuals affected by primary immunodeficiency diseases through advocacy, education, and research.
- Jeffrey Modell Foundation (JMF) - JMF is a global nonprofit organization dedicated to early diagnosis, meaningful treatments, and ultimately cures for primary immunodeficiency diseases. The foundation provides support, education, and advocacy for patients and families affected by these conditions.
- Foundation for Primary Immunodeficiency Diseases (FPID) - Established in the United States to support the education, early diagnosis, genetic counseling, therapy, and research of PID in both India and the United States.
- Advocacy & Awareness for Immune Disorders Association (AAIDA) - Dedicated to patients living with immune dysregulation and overlapping conditions with a focus on advocacy campaigns, educational initiatives, and research opportunities. AAIDA also provides patients and healthcare providers assistance with insurance denials and medication assistance programs available across the United States.
- American Partnership for Eosinophilic Disorders (APFED) - While not exclusively focused on primary immunodeficiencies, APFED provides support, education, and advocacy for individuals with eosinophilic disorders, which can sometimes occur alongside primary immunodeficiency conditions.
- Activated PI3K Delta Syndrome (APDS): A rare primary immunodeficiency that affects approximately 1 to 2 people per million. It occurs when there are variations to the PIK3CD or PIK3R1 genes.
- Primary Immunodeficiency (PID): A group of disorders characterized by defects in the immune system present from birth, leading to increased susceptibility to infections.
- Innate Immunity: The first line of defense against pathogens, involving physical barriers (eg., skin), cellular responses (eg., macrophages, neutrophils), and soluble factors (eg., complement proteins).
- Adaptive Immunity: The immune response mediated by lymphocytes (T and B cells) that specifically targets pathogens and develops memory for future encounters.
- T-Cell Deficiency: A primary immunodeficiency characterized by defects in T lymphocytes, leading to impaired cell-mediated immunity and increased susceptibility to viral, fungal, and certain bacterial infections.
- B-Cell Deficiency: A primary immunodeficiency characterized by defects in B lymphocytes, leading to impaired humoral immunity and increased susceptibility to bacterial infections, particularly encapsulated bacteria.
- Combined Immunodeficiency: A primary immunodeficiency characterized by defects affecting both T and B lymphocytes, leading to severe immunodeficiency and susceptibility to a wide range of infections.
- Severe Combined Immunodeficiency (SCID): A rare and severe form of combined immunodeficiency characterized by profound defects in both T and B lymphocytes, often resulting in life-threatening infections within the first few months of life.
- Antibody Deficiency: A primary immunodeficiency characterized by defects in antibody production, leading to impaired humoral immunity and increased susceptibility to bacterial infections, especially extracellular pathogens.
- Common Variable Immunodeficiency (CVID): A primary immunodeficiency characterized by low levels of serum immunoglobulins (particularly IgG and IgA), leading to recurrent bacterial infections, autoimmune disorders, and an increased risk of malignancy.
- Complement Deficiency: A primary immunodeficiency characterized by defects in components of the complement system, leading to impaired opsonization, chemotaxis, and lysis of pathogens, resulting in increased susceptibility to bacterial infections, particularly Neisseria species.
- Phagocytic Defects: A primary immunodeficiency characterized by defects in phagocytes (eg., neutrophils, macrophages), leading to impaired clearance of pathogens and increased susceptibility to bacterial and fungal infections.
- Hyper IgM Syndrome: A primary immunodeficiency characterized by defective class switching of immunoglobulins, resulting in low levels of IgG and IgA but normal or elevated levels of IgM, leading to increased susceptibility to bacterial infections.
- X-linked Agammaglobulinemia (XLA): A primary immunodeficiency caused by mutations in the gene encoding Bruton’s tyrosine kinase (BTK), resulting in the absence of mature B cells and low levels of immunoglobulins, leading to recurrent bacterial infections.
- Autoimmune Lymphoproliferative Syndrome (ALPS): A primary immunodeficiency characterized by defective apoptosis of lymphocytes, leading to lymphoproliferation, autoimmune manifestations, and an increased risk of lymphoma.
- Hematopoietic Stem Cell Transplantation (HSCT): A procedure in which hematopoietic stem cells are infused into a patient to restore bone marrow function and immune system competence often used as a treatment for severe primary immunodeficiencies.




